Coordinating analytical and stability studies to support non-clinical and clinical trials for biologicals and other medicines.
Introduction
Stability studies are a requirement; they provide data for determining the shelf-life, storage and transportation conditions for:
an investigational medicinal product (IMP) to be used in clinical trials;
the marketing authorisation application for a medicinal product and
the extension of the current shelf-life for an authorised medicinal product.
In this blog post I will discuss my experiences in conducting analytical and stability studies for nonclinical and clinical trials.
Nonclinical studies
The objectives of nonclinical safety studies are to define the pharmacological and toxicological effects not only prior to initiation of human clinical trials but throughout the clinical development.
All non-clinical studies should be conducted to Good Laboratory Practice, and the status of which is confirmed by inspection by an EU Member State or a country that is a member of the Organisation of Economic Co-operation and Development (OECD). Prior to the initiation of any studies, the Quality Affairs department would need to perform their inspections and obtain the documentation to confirm this.
When designing nonclinical studies, the stability of the test material under the condition of use must be established; this is referred to in the ICH guideline ‘Preclinical Safety Evaluation of Biotechnology Derived Pharmaceuticals S6 (R1)’. A protocol describing the experiment designed to demonstrate the stability of and compatibility of the IMP with the administration devices was prepared and approved prior to execution. The data generated in accordance with the protocol, was evaluated and reported before the actual nonclinical study was initiated. Performing this activity after the nonclinical study has concluded is a risky approach not to mention the temptation for confirmation bias when interpreting the data; I have been in those situations. The stability study is always designed to mirror the actual procedures for administration to the concerned animals.
For example, diluted IMP solutions to be given to animals is prepared the night or day before, by diluting with saline, transferring to IV bags and administering to the animals the following day. Therefore, the study was designed to test the dilute solutions by preparing the solutions accordingly and transferring to IV bags the day before; the bags were stored overnight, in a fridge before being delivered, using the exact proposed pumps and devices to be administered to the animals. The time taken for the administration mirrored the actual nonclinical study. The data generated confirmed the compatibility of the active substance with the saline and medical devices used for the animal testing; the data also showed that the diluted solution was stable for the period of use and the purity and biological activity were within the required acceptance criteria.
Some background on nonclinical studies: nonclinical studies should always be performed on relevant animal models. They provide information on the safety, biodistribution and toxicity of the IMP from in vivo and in vitro studies. They should mirror the dosing regimen for clinical trial. Nonclinical studies provide information on proof of concept, route of administration, dosing schedules and potential adverse reactions. For clinical trials that are going straight into patient subjects, nonclinical studies are critical. More information can be found on the ICH website; I will write an overview on non-clinical studies in another post.
Stability studies for Clinical Trials
Characterisation studies determined early in development yields information on the stability of the IMP. Biological materials are generally less stable at ambient temperature compared to small molecules and are usually stored in refrigerated conditions, at least that has been my experience. Stability studies are conducted to support the proposed shelf-life and storage conditions of the IMP during the clinical trials.
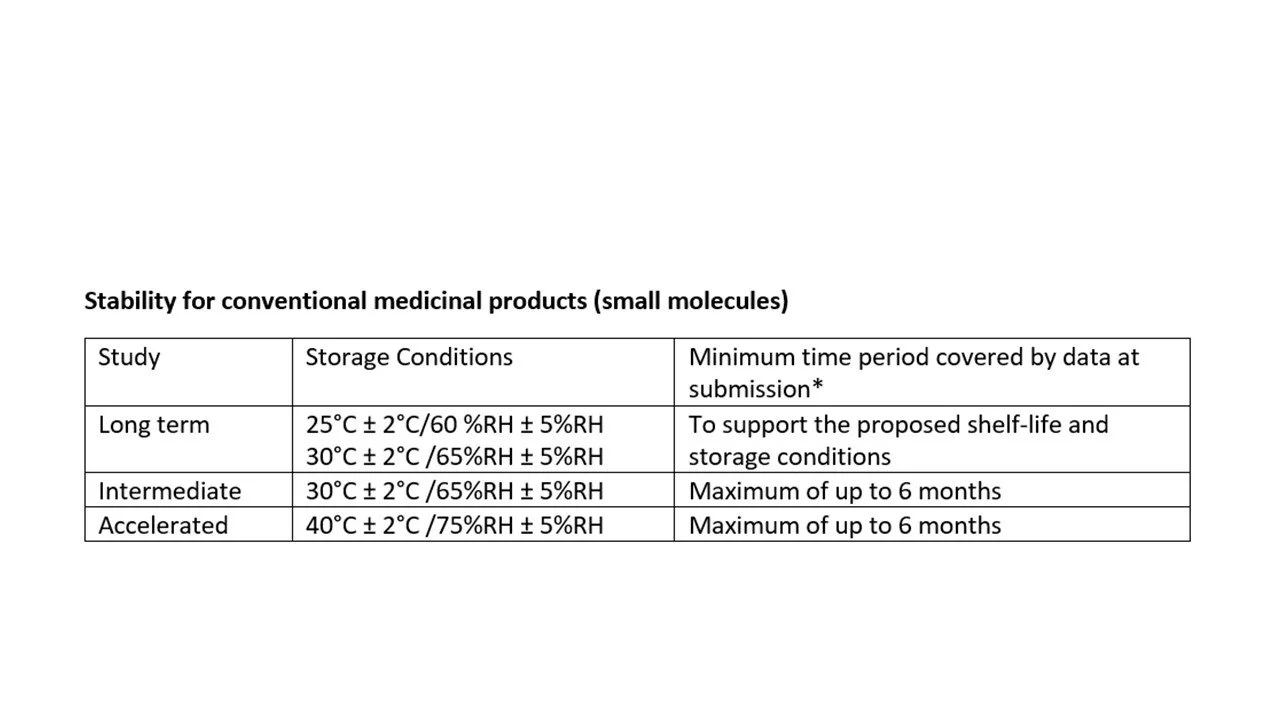
My experience of the design of stability studies is illustrated in the tables below.
Include small molecule active substances
e.g., tablets, oral suspensions, oral solution, capsules etc.
Proteins and antibodies.
The data generated by the accelerated conditions for biological medicinal products were generated to justify temperature excursions particularly since we knew that the IMP was not stable at higher temperatures after 2 weeks. Stress testing at accelerated conditions (such as 30°C ± 2°C /65%RH ± 5%RH and 40°C ± 2°C /75%RH ± 5%RH) and later timepoints indicated as per the ICH guideline were not applicable for the biological products that I have worked on.
Stability studies to support product development
The data generated from stability studies were used to support the storage conditions, re-test period, or shelf-life for the active substance, and/or bulk product / final drug product following manufacture. In addition, stability data was used to justify batch size increases, manufacturing changes, registration of additional manufacturing sites and shelf-life extensions; in these cases, comparative stability data was analysed and presented in applications to demonstrate product comparability.
Later in the development program, a strategy to freeze the bulk drug product (i.e., a change in the manufacturing process that could impact on quality) was proposed. The required data to demonstrate comparability with unfrozen material required a protocol of the proposed analytical exercise. Therefore analytical/stability studies were set up using samples manufactured in accordance with the current process and subjected to the freeze and thaw process, with an additional freeze thaw step; this additional step was not to be performed in real life situation but illustrates the robustness and stability of the product to the freeze thaw cycling. A bioassay test which was not included in previous registered specification, was included alongside the other routine tests in the freeze thaw study, to strengthen the comparability data and support the proposal to freeze the bulk. The data generated supported the change in manufacture process and freeze thaw process was implemented.
In addition to the above, analytical and stability studies were conducted to demonstrate comparability of the bulk drug product and the active substance for the following:
Manufacture at different sites, in different countries.
Manufacturing technology transfer.
Manufacturing changes e.g. batch size increases and changes in the steps of the manufacturing processes.
To support clinical trials in different regions.
The stability data was generated on the ‘bulk’ active substance and the bulk drug product used in clinical trials, i.e., in the final containers (the stoppered glass vials). I also designed and conducted stability studies to look at compatibility with the container closure material, by storing samples in inverted positions or laid on the side, to ensure that real life occurrences did not impact on the quality of the IMP.
Test method and specification
Some of the issues encountered with test methods included the fact that the analytical procedures themselves were not ‘stability indicating’; some methods could not detect degradation products, but they could measure the reduced concentrations of active substances in the stability samples. Other test methods utilised could detect degradation to varying degrees; some methods detected aggregation other test methods was detected changes to the product on storage; this was observed by the presence of other bandings in their pattern profiles when compared to standards (IEF and SDS-PAGE).
Stability of biological IMPs was determined by reviewing the assay content for the active substance by Size Exclusion Chromatography and affinity chromatography, the results of other tests such as SDS-PAGE (for aggregation), changes in pH readings and Isoelectric focussing profiles. The approach taken was to perform a cumulative review of all of the data generated by all of the tests to evaluate the trends and determine the overall stability.
There was an inherent variability with most of the analytical procedures used for these products.
In use stability studies
These studies are mainly to provide evidence supporting the use of the product in the clinical or home setting. Therefore, materials such as saline, and medical devices such as IV bags, pumps, and tubes, and/or injection kits etc. are included in these studies. The studies mirrored exactly how the dose was administered with the same devices and the maximum administration times.
Stability data for Investigational Medicinal Product Dossier
The data to support the quality of the IMP after storage within the shelf-life period and transportation under controlled conditions, must be generated, reviewed, and included in the Investigational Medicinal Product Dossier (IMPD) for a clinical trial authorisation.
The testing period and frequency is very much determined by the stability of the biological product. For example, for some biological products initial testing and testing at 1 month, 2 months, 3 months is performed; then a decision is made based upon the data generated as to whether or not testing should be conducted at 6 months or 9 months etc. until an understanding of the stability profile of the biological product is attained. This information is then applied to subsequent batches placed on stability.
Other biological products are only stable for a number of hours or days and therefore the stability study was adjusted accordingly.
In the case of the biological material, it was possible to place 3 batches on stability. For ATMPs - I know that this is not always possible or ethical since all of the material may be required for the patient; in such scenarios representative batches from healthy subjects could be employed, and adjustments factoring in the differences between healthy subjects and diseased patients would need to be made (prior justification by way of scientific advice is recommended).
Stability studies for Marketing Authorisation Application
The data generated for clinical trials are used for MAA. This includes data from stability studies generated during the development, demonstration runs, exhibit and clinical trial batches etc. Comparability studies to show that data generated on the nonclinical and previous clinical trials are applicable to manufacture for commercialisation should be used to determine shelf-life and storage conditions.
For MAAs, if the scale or batch size of materials used to determine the shelf-life and storage conditions are different, to the proposed commercialisation scale (if a validation manufacturing protocol were provided for the MAA application, instead of validation data), most likely a commitment to place the first three commercial batches on stability and provide the data (or at least assurances that there are no out of specification or potential out of specification at later time points) is an expectation – certainly this approach is taken for small molecules and biologics. As I mentioned above, for rare diseases where the medicinal products are ATMPs, some authorities expect the manufacturing scale for a MAA to be representative of that required for the total patient population; at least that is what I have been advised during a seminar that I attended with an FDA assessor giving the presentation.
The amount of material manufactured during a campaign is dependent on the clinical trial requirements; it is also dependent on the proposed manufacturing scale and conditions for commercialisation.
Conclusion
Stability studies are used for the application of a re-test period or shelf-life and storage conditions for active substance(s) and the drug product. Statements on labelling of the medicinal products, relating to shelf-life and storage conditions are a legal requirement. This information is included in the dossier and in the pharmaceutical section of the Summary of Product Characteristics and the labelling (the patient information leaflet and the container labels).
Stability studies is an important function for the authorisation of medicinal products.
I hope you found my post interesting.
Best wishes…